First US Cultivated Seafood Approval, Mycoprotein from Ethanol Waste, and Cocoa Biomanufacturing Scales Up
Also: University of Oxford and Wild Bio to receive £6.7M grant to improve potato and wheat yields using new synthetic biology approach.
Hey, welcome to issue #106 of the Better Bioeconomy newsletter, your weekly dose of biotech’s latest in food and agriculture. Thanks for being here! 👋🏾
History was made last week as Wildtype became the first startup cleared to sell cultivated seafood in the US. Nordic Foodtech VC raised €40M to back next-gen food tech. Enifer and FS are scaling ethanol-to-protein fermentation in Brazil, and UK scientists secured £6.7M to boost yields via chloroplast engineering. And global Islamic scholars gave the green light to halal cultivated meat under specific conditions.
Let’s dig in!
BIO BUZZ
Products, partnerships, and regulations
🇺🇸 Wildtype becomes the world’s first startup to get US regulatory approval to sell cultivated seafood
San Francisco startup Wildtype has made history as the first company to get FDA approval to sell cultivated seafood in the US. Its sushi-grade cultivated coho salmon is now being served at Kann, the Portland restaurant led by James Beard Award winner Gregory Gourdet.
The approval took about three years and involved eight rounds of updates and reviews. The FDA concluded that Wildtype’s cultivated salmon is “as safe as comparable foods produced by other methods,” which means the company can now sell it to restaurants.
Wildtype’s process involves culturing salmon cells in bioreactors with a precise blend of nutrients and conditions. After harvesting and refining, the cells are combined with plant-based components to recreate the appearance and mouthfeel of raw salmon. This approach provides a novel way to meet the demand for popular fish, such as coho salmon, some of which are endangered.
Source: Green Queen
🤔 Thoughts:
Wildtype’s approval is a much-needed proof point at a time when the cultivated meat and seafood sector is navigating financial strain and growing political resistance.
It also highlights an increasingly fragmented regulatory landscape in the US: while the FDA affirms the safety and legitimacy of cultivated seafood, several states are moving to restrict or ban these products altogether. Wild times.
Tangible milestones like this reassure investors that the technology can move from lab to market, and signal to the public that the “future of food” isn’t always a decade away.
It also addresses a significant logistical barrier, proving that a startup can navigate the whole regulatory process, which may streamline things for followers or at least provide a template.
🇫🇮🇧🇷 Enifer partners with Brazilian ethanol giant FS to upcycle corn ethanol byproduct into sustainable mycoprotein
The Finnish startup and FS are constructing a 500-ton pilot facility in Brazil, backed by R$9.8M from the innovation agency FINEP. The aim is to test how well Enifer’s PEKILO process fits into corn ethanol production and gauge local market interest. If all goes well, they’re looking at scaling up to 10,000 tons per year, creating a new regional supply of sustainable protein.
PEKILO is a neutral-tasting, protein- and fibre-rich mycoprotein, initially aimed at aquaculture and pet food in Brazil, Ecuador, and Chile. Down the line, the company plans to explore uses in human foods like meat alternatives and snacks.
Instead of traditional methods that dilute protein content, the PEKILO process turns glycerol, a low-value component of thin stillage, into concentrated protein, creating a more efficient use of ethanol byproducts for animal nutrition.
Source: AgFunder
🤔 Thoughts:
This partnership fits into a growing trend toward integrated biorefineries and circular bioeconomy models. By showing an “alternative, higher-value route” for ethanol byproducts, Enifer and FS are validating a model where one industry’s leftovers become another’s raw material on an industrial scale.
The timing couldn't be better. Brazil's corn ethanol production has exploded to 6 billion liters in 2023-24, an 800% surge in just five years, creating a steady supply of underused feedstocks like thin stillage. With that kind of input and infrastructure already in place, we’re likely to see more cross-industry projects that blur the lines between food, fuel, and feed, as existing facilities are adapted for multi-product manufacturing.
🇺🇸 California Cultured successfully scaled from laboratory-scale to large-scale biomanufacturing of cell-based cocoa
This milestone demonstrates that cellular agriculture can move beyond research phases to potentially become a viable solution for producing commodity foods, particularly those facing environmental and supply chain vulnerabilities like cocoa.
The startup partnered with Berkeley-based Pow.Bio to leverage Pow.Bio’s newly commissioned 25,000 sq ft biomanufacturing facility, which is equipped with dual-chamber continuous fermentation systems, clean room capabilities, and real-time AI-based process control.
The production process, from cell cultivation to harvest, takes just 3–4 days, a much shorter timeline than traditional cocoa farming. This could be a practical way to ease some of the strain on the chocolate industry as it deals with climate risks and rising prices.
Source: Green Queen
🤔 Thoughts:
California Cultured’s transition from lab-scale to large-scale bioreactor fermentation tackles one of the biggest hurdles in cellular agriculture: scaling up. With cocoa prices hitting record highs in 2024, up 136% in under two years, the timing couldn’t be more relevant.
What also stands out is the role of Pow.Bio. The emergence of specialised ‘biomanufacturing-as-a-service’ infrastructure could accelerate the entire novel food/future food sector by providing shared infrastructure, designed around food-grade, not pharma-grade, economics.
Instead of each startup building its own (likely) CAPEX-heavy facilities, this allows them to connect with specialised platforms and work with process experts to accelerate scale-up. It’s a step toward an ecosystem where more asset-light cellular agriculture companies can emerge and grow faster.
🇶🇦 The International Islamic Fiqh Academy ruled that Muslims can consume cultivated meat if certain conditions are met
At the 26th IIFA Conference in Doha, the International Islamic Fiqh Academy (IIFA), a key global Islamic authority, declared that cultivated meat is permissible for Muslims if it meets religious and ethical standards. This could potentially open cultivated meat to ~2 billion Muslims worldwide.
To be considered halal, the source cells must come from animals allowed under Islamic law and properly slaughtered. The cultivation process must also avoid prohibited substances like blood (including fetal bovine serum), and be developed under trustworthy regulatory supervision.
The event brought together 230 experts from over 60 countries discussing contemporary issues, like AI, genetically modified foods, and cultivated meat. Genetically modified foods are similarly permitted under Islamic law, provided they are made from halal animals, processed in a safe and Sharia-compliant way, and include full disclosure about their preparation.
Source: Green Queen
🤔 Thoughts:
The IIFA’s ruling isn’t just a green light for Muslim consumers, it’s a signal that cultural legitimacy is becoming a strategic differentiator in food tech, like regulatory approval.
Companies that proactively incorporate religious and cultural requirements into their process like developing serum-free media, obtaining scholarly endorsements are positioned to capture first-mover advantage in untapped Halal markets. It’s also a way to build trust in places where public acceptance depends as much on moral credibility as it does on scientific backing.
🇩🇪 Bayer developed new tomato varieties with multi-stacked genes to offer protection against resistance-breaking fruit virus
The hybrids combat both standard and resistance-breaking strains of ToBRFV (Tomato Brown Rugose Fruit Virus), addressing the long-term threat of resistance-breaking ToBRFV to grower incomes by providing longer-lasting solutions that maintain produce quality and agronomic performance without sacrificing commercial viability.
In two glasshouse trials, four new hybrids were tested under high virus pressure. While non-resistant plants showed severe symptoms by day 14 and 21 after inoculation, the multi-stacked varieties held up against both the standard and resistance-breaking strains of ToBRFV.
The new hybrids will roll out in 2025 across all major glasshouse tomato categories like Large Truss, Medium Truss, Cocktail & Cherry Plum Truss, and Beef. Some, like the De Ruiter red beef Ferreira and pink beef Futumaru, are already on the market.
Source: Bayer
🤔 Thoughts:
The move from single-gene resistance to multi-stacked architectures is part of a growing trend in crop protection: designing resilient systems, not just resilient genes. It's similar to what we see in cybersecurity and pharmaceuticals, where layered defences have become the norm when dealing with rapidly evolving threats.
Bayer’s choice to pressure-test its ToBRFV-resistant tomatoes against a breakthrough mutant shows the shift from reactive breeding to anticipatory design: creating varieties meant to delay, and ideally stay ahead of, viral evolution instead of scrambling after each new outbreak.
⚡️ More buzzes
🇦🇺 Australian cultivated meat startup Magic Valley held a cultivated meat tasting for 17 politicians and ministers in the New South Wales parliament, serving lamb meatballs and pork dumplings that were well-received. One politician called it "delicious" and "guilt-free: no animal cruelty, no deforestation, and saves water and CO2 emissions.” (Green Queen)
🇦🇹🇺🇸 BioCraft's cultivated mouse meat emits 92% less emissions than beef byproducts in pet food. The Austrian-American startup's cultivated mouse meat generates just 1.73kg of CO₂ per kg, while conventional beef byproducts emit 21.28kg. The analysis, conducted by ClimatePartner, followed established methods like the GHG Protocol and accounted for all major greenhouse gases as CO₂ equivalents. (Green Queen)
🇺🇸 California has created the Assembly Select Committee on Alternative Protein Innovation, chaired by Ash Kalra, a Democrat from San José, to reinforce its leadership in sustainable food systems and support its thriving alternative protein startup ecosystem. (Green Queen)
CLIMATE FEED FELLOWSHIP
📢 Green Queen Media launched the first-ever fellowship dedicated to empowering early-career journalists to tell a better food and climate story
Food and agriculture are responsible for up to one-third of global greenhouse gas emissions, but the media rarely links them to climate change. Despite the high environmental impact, food systems remain a blind spot in climate reporting.
To change this, Green Queen Media launched the Climate Feed Fellowship, a much-needed program offering 6 early-career journalists a 12-week, guided opportunity to create compelling stories that bridge food and climate issues.
Fellows receive:
Editorial mentorship from leading industry editors
Access to expert-led workshops and panels
A platform to publish their work on Green Queen’s leading global media platform
A stipend for their contributions
Open to journalists, freelance writers, and reporters worldwide with up to 5 years of experience. Apply by June 20!
BIO BUCKS
Funding, M&As, and grants
🇫🇮 Nordic Foodtech VC raised €40M in the first close of its second fund, aiming for a final close of €80M
The fund is backed by institutional investors like Tesi and Elo Mutual Pension Insurance Company, along with food industry players such as Valio Pension Fund and Heino Group. It invests in early-stage B2B food and agtech startups in the Nordics and Baltics, with selective co-investments elsewhere in Europe.
Writing checks between €500k and €2M, Partner Lauri Reuter says their focus is on backing sustainable, strongly differentiated tech rather than chasing unicorns, given the more grounded exit landscape in food tech compared to traditional tech VC models.
They work closely with universities to tap overlooked innovations from research institutions, targeting ingredients with high value and low usage rates like aromas and haemoglobin-like proteins (citing their portfolio companies Evodia and Ironic Biotech), while identifying other high-value opportunities like lactoferrin, for more immediate commercial viability.
Source: AgFunder
🇬🇧 University of Oxford and Wild Bio to receive £6.7M grant to improve potato and wheat yields using new synthetic biology approach
Backed by the UK’s Advanced Research and Invention Agency (ARIA), the OPTIMiSE project aims to redesign the chloroplast genome, an area previously untapped by traditional breeding. This effort seeks to improve critical traits like photosynthesis, stress tolerance, and nutrient efficiency using in vitro DNA assembly.
Their precision breeding approach creates "designer" crops that align with international definitions of precision-bred organisms rather than GMOs, making them more regulation-friendly and acceptable to farmers and policymakers globally.
Starting with potato and moving on to wheat, the project targets two of the world’s top three staple crops to develop varieties that can produce more food with fewer resources under challenging conditions like drought and extreme heat.
Source: University of Oxford
GEEK ZONE
Latest scientific research papers
🌸 Engineered yeast hits record-high production of plant-derived β-caryophyllene, a high-value flavour compound
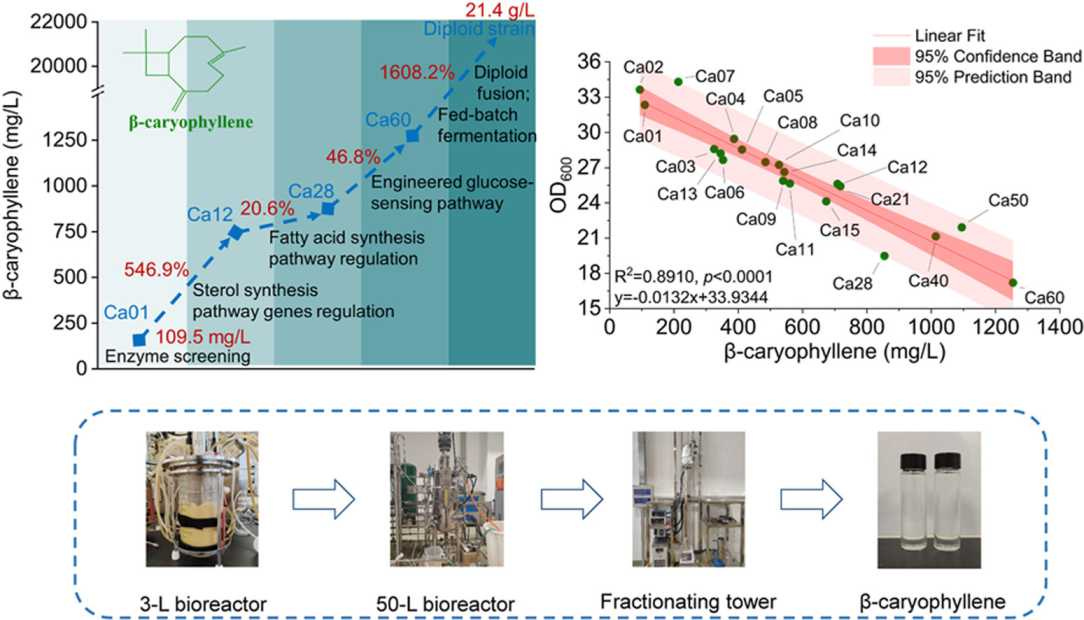
Researchers reprogrammed yeast to increase production of β-caryophyllene by replacing native gene promoters with glucose- and sterol-sensitive ones, thereby redirecting resources away from competing pathways and toward the production of the target compound.
They pushed production even further by fusing cells into a diploid strain and fine-tuning fed-batch fermentation. Production increased from 854.7 mg/L (a 6.8× boost) to 1.25 g/L with pathway control, and peaked at 21.4 g/L, which is said to be the highest β-caryophyllene titer reported to date.
β-caryophyllene is a high-value sesquiterpene, but plant extraction remains expensive and inefficient. This breakthrough microbial production platform could enable cost-competitive biosynthesis at an industrial scale.
Source: Metabolic Engineering
🧬 Engineered E. coli increased the natural sweet protein thaumatin production by 45% with improved solubility
Researchers used E. coli to produce thaumatin II, a natural sweet protein, and tackled its low solubility by testing three improvement strategies. The most effective approach combined molecular chaperones for better folding with directed evolution, leading to an optimised mutant called M-882.
M-882 reached a titer of 42 mg/L, up 45% from the original, thanks to structural changes that reduced mispairing during peptide folding and improved solvent access, all while keeping its sweetness intact.
Improving microbial production of sweet proteins like thaumatin could enable scalable, low-calorie sugar substitutes for food and beverage manufacturers. This work provides a blueprint for bioengineering other functional proteins for food applications.
Source: Journal of Agricultural and Food Chemistry
🥬 Root-dwelling fungus increased leafy green yields by 42% through auxin signalling and phenylpropanoid reprogramming
Researchers found that Tinctoporellus strain AR8, a root endophyte in Brassica crops like choy sum and kailan, boosts plant growth by triggering the plant’s auxin production and rewiring the phenylpropanoid pathway, central to lignin formation and hormone signalling.
In both lab and field settings, AR8 raised shoot biomass by 37–42%, driven by increased phenylpropanoid activity (especially p-coumaric acid). Blocking key steps in the phenylpropanoid (PAL1) or auxin (YUC589) pathways removed this effect, but biomass gains were restored in mutants through p-coumaric acid complementation.
The AR8–plant interaction maps a biochemical route (phenylpropanoid–auxin axis) that points to a biofertilizer opportunity using native root fungi to boost yields without genetic modification or synthetic inputs.
Source: Cell Reports
EAR FOOD
Podcast episode of the week
🎧 How Corteva’s ‘Genlytix’ gene editing ecosystem accelerates sustainable agriculture
Host: Tim Hammerich
Guest: Reza Rasoulpour, Heather Hampton Knodle
Gene editing tools are widely accessible, even small startups and academic labs can use them. But Corteva’s edge lies in its deep data library: almost a century’s worth of corn and soybean pedigree, performance, and environmental data. Combined with AI and machine learning, it lets Corteva pinpoint “where to edit,” giving it an edge over labs that can edit but lack large proprietary libraries.
Since 2013, Corteva has been using gene editing tools, and now it’s bringing everything under one roof with its “Genlytix” platform. It’s more than a tech stack. It’s a way to unify internal R&D, external partnerships, and stewardship into a coordinated, collaborative approach.
One standout result is a multi-disease-resistant “super locus” in corn. Through gene editing, Corteva stacked resistance to four major pathogens- grey leaf spot, northern leaf blight, southern rust, and Anthracnose- into a single edit. It could help prevent losses exceeding US $1 billion per year. Field plots show green, healthy plants beside heavily diseased controls.
Gene editing also poses a massive data challenge. Corn alone has 2.5 billion base pairs. That’s where AI and machine learning come in. Such tools could identify the optimal editing sites, predict outcomes, and enable more precise and scalable breeding.
Despite technical progress, the bottleneck for the adoption of gene editing remains global regulatory alignment. Export markets need to approve gene-edited crops to avoid trade friction. Progress is underway in the Americas, EU, and APAC, but uneven and slow.
GOT A MINUTE?
If you found value in this newsletter, consider sharing it with a friend who might benefit! Or, if someone forwarded this to you, consider subscribing.
This newsletter is free, but if you'd like to support the time and effort behind each issue, a small pledge is always appreciated.
Thank you, and have a great day!
Disclaimer: The views and opinions expressed in this newsletter are my own and do not necessarily reflect those of my employer, affiliates, or any organisations I am associated with.




Hi Eshan! How are you doing on this very nice day? Thank you so much for excellent issue #106. There are many positive developments you highlighted in this important issue, very cool! Today, I saw a video of this building in China, which is the largest factory pig farm in the world.
https://www.nytimes.com/2023/02/08/business/china-pork-farms.html?smid=url-share
A big bucket list item for me is to live long enough to see cultivated meat become so good, at a substantially lower cost than factory farming, that we see less of these Chinese factory farms. The community appreciates all your hard work and efforts you put into helping educate us about the latest developments in the alt protein space. Have a wonderful week my friend 👍❤️
> The International Islamic Fiqh Academy ruled that Muslims can consume cultivated meat if certain conditions are met
I wouldn't take this that seriously. Sunni Islam isn't like the Catholicism. There has never been and never will be a consensus view on the interpretation of Islamic law. Hence there will be a lot of heterogeneity in whether Muslims will actually accept the technology.