How ‘Tricking’ Cells Into High Production Can Transform Biomanufacturing
What if microbes in a bioreactor had only one rule: make the product or perish?
Hey folks!
Thanks for being here. In Issue #115 of Better Bioeconomy, I want to explore a technology that essentially “tricks” microbes into staying productive by linking survival to output. Enduro Genetics is developing this technology, and I believe it’s one of the more intriguing approaches in industrial biotech right now.
I first heard about Enduro earlier this year when they announced their €12M Series A, and since then, I’ve been fascinated by their technology. Today, I want to take a closer look at how Enduro’s platform works and then zoom out to what this breakthrough could mean for biomanufacturing. To be clear, I have no affiliation with the company. I just really like their premise.
As always, my understanding keeps evolving as I dig deeper. If you spot something I’ve missed or got wrong, let me know. Let’s jump in!
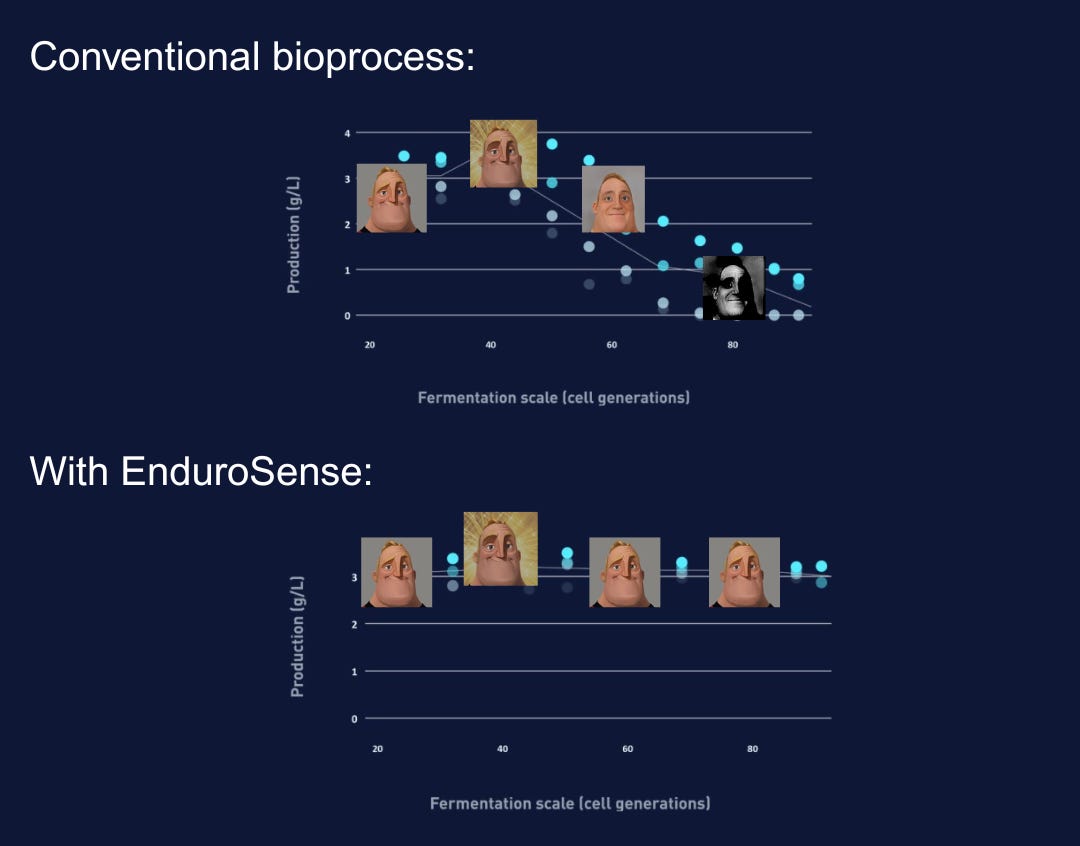
Imagine you’re running a bioreactor brewing dairy proteins (like whey or casein) using engineered microbes. At first, your microbes churn out the product as intended. The tank looks healthy, the output rising. But after a few days (about 60-80 microbial generations), something happens. The yield plateaus and then plunges.
You sample the broth and discover the uncomfortable truth: many of your microscopic workers have gone on strike. Only a fraction of the cells are still producing the target compound. The rest have quietly mutated into “freeloaders,” gobbling up sugar and nutrients while contributing nothing useful. The thing is, in industrial biotech, this isn’t a freak accident. It is common in long(er) fermentation runs.
Given the chance, a microbe will prioritise its own survival over our plans for production. Higher-producing cells carry a heavier metabolic burden, so any cell that drops the production genes can grow faster and outcompete its diligent neighbours. Left unchecked, these slackers take over the reactor, and your process grinds to a halt. It is evolution working against engineering. A persistent challenge across biomanufacturing.
How do you stop these evolutionary freeloaders (non-producers) from undermining production? In fermentation facilities, operators have tried various approaches, such as antibiotic resistance markers, to maintain plasmids. Yet the cells still find a way out. By the end of a long run, you often end up with a diverse mix of mutants, with only a small minority still making the product.
Back in my lab days (I feel like an old man saying this), I kept running into the same pattern. We could program a microbe to make almost anything, yet keeping it productive for longer durations was the hard part.
Enduro Genetics thinks it has a solution. They’re flipping the script on evolution, effectively addicting microbes to the act of production. In the next sections, I’ll dig into how their platform works, and then zoom out to what this breakthrough could mean for biomanufacturing.
A genetic plug-in that flips evolution in favour of production
Spun out of the Technical University of Denmark, Enduro’s premise is straightforward: if evolution keeps making non-producers win, then change the game so that only producers can survive. In practice, Enduro’s technology “couples each cell’s productivity to its health and growth”.
Instead of evolution favouring the freeloaders, Enduro’s genetic system makes high production the ticket to survival. If a cell stops producing the target compound, it loses a survival advantage and eventually gets outcompeted and eliminated. The result is a kind of built-in quality control where, over time, your bioreactor enriches for the hardest-working cells instead of the laziest.
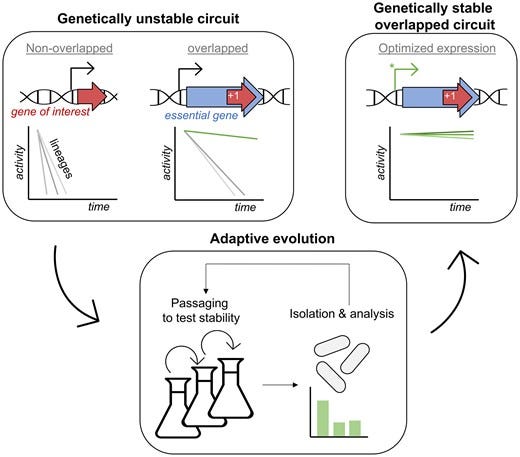
How exactly do they achieve this? The core product is called Enduro Sense, described as a “genetic plug-in” for production strains. It’s essentially a biosensor circuit inserted into the microbe’s genome. This circuit links the expression of an essential gene (one the cell can’t live without) to the presence of the target product. In other words, the microbe is genetically wired such that it must keep making the product (or a key intermediate of it) in order to activate a gene needed for its own survival.
Only cells that continue churning out the product get to grow and divide, because the sensor turns on the vital gene in proportion to product output. If a cell “decides” to slack off, say, a mutation knocks out the production pathway, the sensor circuit doesn’t trigger the essential gene strongly enough, and that cell’s lineage will slow down or die off.
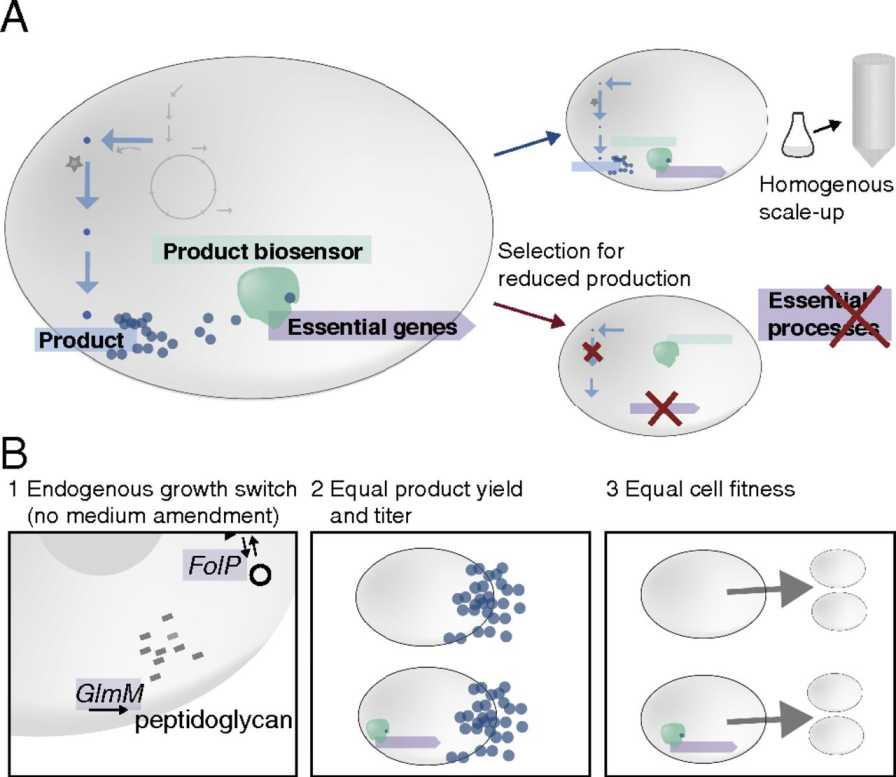
In the scientific literature, this concept is called “synthetic addiction.” Dr. Peter Rugbjerg, Enduro’s co-founder and chief scientific officer, pioneered it through years of research at the Technical University of Denmark. In one landmark study, Rugbjerg and colleagues engineered E. coli bacteria to produce mevalonic acid while implementing a synthetic addiction circuit. They tied an essential growth gene operon to a mevalonic-acid-responsive promoter.
The outcome was dramatic: the “addicted” bacteria kept high production going for 95 generations, roughly equivalent to the duration of an industrial 200,000-litre fermentation, whereas a normal strain had virtually stopped producing by that point.
From proof-of-concept to platform
Enduro Sense takes this principle and makes it into a platform that can be applied to different microbes and products. According to Enduro’s CEO, Christian Munch (a Novozymes veteran with 14 years in industrial enzymes), the team has tested the system in E. coli, Bacillus strains, baker’s yeast, and filamentous fungi, producing everything from milk proteins to antibody fragments.
This broad applicability is important: it suggests Enduro’s approach isn’t limited to a single niche, but could function as a universal add-on for bioproduction strains. Also, the technology is product-independent, meaning the biosensor circuit can be adapted to many target molecules. Enduro maintains a library of sensor parts and an in-house design algorithm to develop a custom “addiction module” for each new project.
Another benefit is that adopting Enduro Sense doesn’t require overhauling the existing process. It’s a one-time genetic tweak to the production strain (typically at the master cell bank stage). Critically, the company notes it needs no foreign genes (such as antibiotic-resistance markers) and requires no special additives or media changes.
In other words, the process runs just as it usually would: same feed and same tank, except the cells have this new internal circuit keeping them in line. This makes it relatively straightforward for a biomanufacturer to try Enduro’s solution. They can send their strain to Enduro, or Enduro can send the DNA construct to them to integrate in-house.
Either way, once the strain carries the addiction circuit, it should behave better in large-scale fermentation. Enduro reports that successful implementations have yielded 30% or more improvement in titer and yields, and fermentations that run five times longer before productivity declines. Such gains can be game-changing. A 30% boost in yield might turn an unprofitable process into a profitable one, and being able to extend runs means fewer shutdowns and more product from the same equipment.
To put it simply, Enduro’s platform tricks evolution into working for us rather than against us. In Munch’s words, they “reverse the selective pressure” so that producing cells, instead of mutants, have the edge. It’s as if the microbes have been reprogrammed with a new prime directive: make the product or perish.
Risks
Clearly, I’m optimistic about Enduro’s approach, but it’s not a panacea. In my view, these are the potential practical failure modes and integration frictions to consider. (Note: Assessments are based on public sources, not direct Enduro data or interviews. Given the team’s background, it’s likely many of these challenges are already being addressed.)
1. Circuit tuning & biosensor reliability
Addiction only works if the sensing-and-control loop is well balanced. Too strict, and you risk losing cells that are temporarily low producers (e.g., cells passing through stress or poor micro‑environments), hurting volumetric productivity. Too lenient and freeloaders slip through.
Whole‑cell biosensors also carry familiar pitfalls, leakiness, limited dynamic range, and cross‑reactivity, which can generate false positives/negatives and enrich the wrong variants unless carefully engineered and screened.
In practice, engineers usually adjust the “volume knob” (promoter strength, sensor design, feedback) until the signal is stable enough, but it’s not usually plug-and-play.
2. Evolutionary escape routes
Even with careful design, evolution tends to find loopholes. Cells can mutate sensor components, rewire regulatory nodes, or find metabolic bypasses that decouple growth from production.
Reviews of circuit stability document common failure modes, mutations in promoters or regulators, transposon insertions, recombination across repeats, and show that “mutant escape” is a recurring theme in long runs. Strategies like building redundancy or spreading the load across multiple genes can reduce the odds, but they may not eliminate them.
3. Public goods and cross‑feeding
If the sensor is tied to a product that leaks out of cells, there’s a risk that freeloaders can “borrow” it from their neighbours and survive without contributing. That’s the classic public‑goods problem in microbial systems, where one strain exploits a shared resource produced by others.
One way around this is to couple survival to an intermediate metabolite that stays inside the cell, but this adds design complexity and must be tuned on a case-by-case basis.
4. Growth-production trade-offs
Addiction aims to let producers win, but high production still carries resource costs (ribosomes, ATP, membrane capacity). If coupling increases the growth penalty for on‑target production, you can preserve per‑cell output but depress population growth, blunting volumetric productivity (g L⁻¹ h⁻¹).
Process design must verify the net effect on TRY (titer, rate, yield), not just the percentage of producing cells. Growth laws and burden studies underscore how easily overexpression tips a culture into slower growth.
How “addicted” microbes could transform biomanufacturing
If coupling survival to productivity becomes standard practice, here is how it could shift the landscape across operations, economics, and scale-up:
1. Longer runs compound output
By eliminating the usual drop-off in output due to mutant “freeloaders,” producers can extend fermentation runs far beyond current limits. Traditionally, genetic instability has forced companies to stick to shorter batch or fed-batch processes since continuous fermentation is often limited by the appearance of non-producer cells, which eventually crashes productivity.
In trials, fermentations with Enduro Sense have run five times longer with sustained output. Longer, flatter production runs mean each bioreactor yields more product per cycle, with fewer shutdowns for cleaning and turnaround. Over a year, this compounds into a major increase in asset utilisation and directly feeds into both cost and reliability gains.
2. Economics tilt in your favour
Stability at scale also changes the economics. If every cell in the tank pulls its weight, you get more product from the same inputs, directly lowering Cost of Goods Sold (COGS). Christian Munch, Enduro’s CEO, notes that clients see >30% titer improvements in optimised processes, effectively increasing output without changing the input. All the sugar, media and energy that would have gone into feeding deadweight cells is instead converted into product. This efficiency can be game-changing for economics.
Higher productivity per batch means fewer batches (or smaller tanks) are needed to meet demand, so companies might avoid new fermentor purchases or facility builds they once thought necessary. This capital benefit compounds with the longer run lengths described earlier.
3. Scale-up becomes more predictable
One of the hardest things in industrial biotech is translating lab results to factory scale. Yields that look great in a shake flask can falter in a 100,000-litre tank due to shifts in conditions and the emergence of mutants. Addiction circuits could narrow this lab-to-plant gap by enforcing genetic stability.
The process becomes more deterministic and repeatable. This reliability reduces the risk of batch failures or surprise shortfalls in titer, which are expensive and disruptive. Moreover, a stable, high-producing population means less batch-to-batch drift over time. All of this de-risks scale-up.
Consistent output also streamlines downstream processing: if titer and impurity profiles don’t fluctuate wildly from batch to batch, purification trains can be designed and run more efficiently.
4. Tougher hosts and rougher feeds become viable
Tying fitness to production could expand the menu of microbes and feedstocks that are viable at scale. Today, companies mostly rely on a few domesticated workhorse strains (like E. coli or yeast) and relatively refined feedstocks. More exotic or stress-tolerant organisms like extremophiles, soil bacteria, or wild yeasts, often get ruled out because they mutate or drop engineered pathways under industrial conditions.
An addiction circuit would act as a genetic disciplinarian, making sure even these unconventional hosts can’t easily abandon their production duties. That means we could start using microbes that thrive on cheap, non-food substrates (think agricultural residues, waste gases, or brackish feedstock) without fearing that they’ll “go rogue” genetically. New tools for the domestication of wild microbes and using cheaper feedstocks are key to breaking the scale-cost paradox in fermentation.
Enduro’s approach could be the compatibility layer that makes those wild strains and crude inputs workable, keeping tough microbes on task in harsher, less controlled environments. This broadens the palette of bioproduction: producers could choose the best microbe for the job (or the cheapest raw material available) and trust that productivity won’t collapse under suboptimal or large-scale conditions.
5. Less waste, smaller footprint
When fewer resources are squandered on feeding non-producing cells or recovering from failed batches, a bioprocess's environmental footprint reduces. Every point of yield improvement means less sugar, water, and energy per kilogram of product.
Enduro’s team highlights that their tech diverts feedstock and nutrients that would otherwise go into wasted biomass to produce the desired product instead. In practical terms, less agricultural input is needed, and less biowaste is generated for the same output. It directly boosts resource efficiency, which translates to lower carbon emissions for the process.
6. Built-in process control from inside the cell
Addiction circuits do more than suppress freeloaders, they could also turn product-responsive promoters into live reporters of pathway flux. That makes them an internal form of PAT (Process Analytical Technology): firms can use the cell’s signal to tune feed rates, aeration, induction, and temperature in real time, holding populations in the productive “green zone.” Unlike external proxies such as DO (dissolved oxygen) or pH, the genetic readout anticipates drift upstream, reducing oscillations and off-spec batches.
The bigger effect is reproducibility. Over time, this enables a shift from static recipes to model-predictive control, cutting development cycles and de-risking tech transfer. External sensors don’t go away, but the biosensor layer makes fermentations more self-reporting.
Final thoughts
Enduro’s “synthetic addiction” represents a shift in how we think about biomanufacturing: instead of constantly battling evolution, why not rewire it to work in our favour? If the approach proves robust across different microbes, products, and scales, it could become part of the ‘invisible infrastructure’ that makes industrial biology more predictable, economical, and sustainable.
Of course, the story is still unfolding. The technology will need to prove itself in a variety of real-world fermenters, not just in controlled pilot runs. And as with any new tool, adoption will depend on how smoothly it fits into existing regulatory and operational pipelines.
For me, the real excitement isn’t about whether one company can “solve” freeloading cells once and for all, but about how each incremental breakthrough reshapes our expectations of biology as a manufacturing platform.
If you found value in this newsletter, consider sharing it with a friend who might benefit from it! Or, if someone forwarded this to you, consider subscribing.
APAC AGRI-FOOD INNOVATION SUMMIT
Don’t miss your chance to attend the Asia-Pacific Agri-Food Innovation Summit and get 10% OFF with my network code. From breakthrough food systems to next-gen agri-tech, this is where Asia’s innovation leaders connect, collaborate, and create impact.
👀 Use my exclusive partner code for 10% OFF your pass: BIO10
Learn more: https://tinyurl.com/s9k5x3d7
See you in Nov!
Disclaimer: The views and opinions expressed in this newsletter are my own and do not necessarily reflect those of my employer, affiliates, or any organisations I am associated with.



Hi Eshan! How are you doing on this very nice day? Thank you so much for awesome issue #115. I learned more about the process and difficulties of creating cultured food cells in this fantastic newsletter than I had previously known. Wow. I was a philosophy major at UCLA and went to law school. Thanks for staying on top of this great ' re-engineering evolution' development. Both NASA and ESA have recently sent super sophisticated spacecrafts, with incredibly complex trajectories to Jupiter's active moon, Europa. Now If humanity would invest the brainpower and resources into creating a real, sustainable cultured meat as we put into our amazing space probes, we would probably already have greatly reduced factory farming. But there is much headwind. Thank you very much for all you do to help educate your subs and helping to make the world a better place. Have a very nice and peaceful week Eshan. I appreciate you 🌞 ❤️
This is excellent, thanks Eshan! What an encouraging technology. Is there any reason this couldn’t work with Cyanobacteria? Thinking of bloating high-lipid strains to dial up the production of oils, or even insulin production a la Cyanocapture.
Also, for any microbes that “beat” the addiction through mutation— is the idea to have those strains serve as case studies that could ultimately strengthen the addiction circuit?